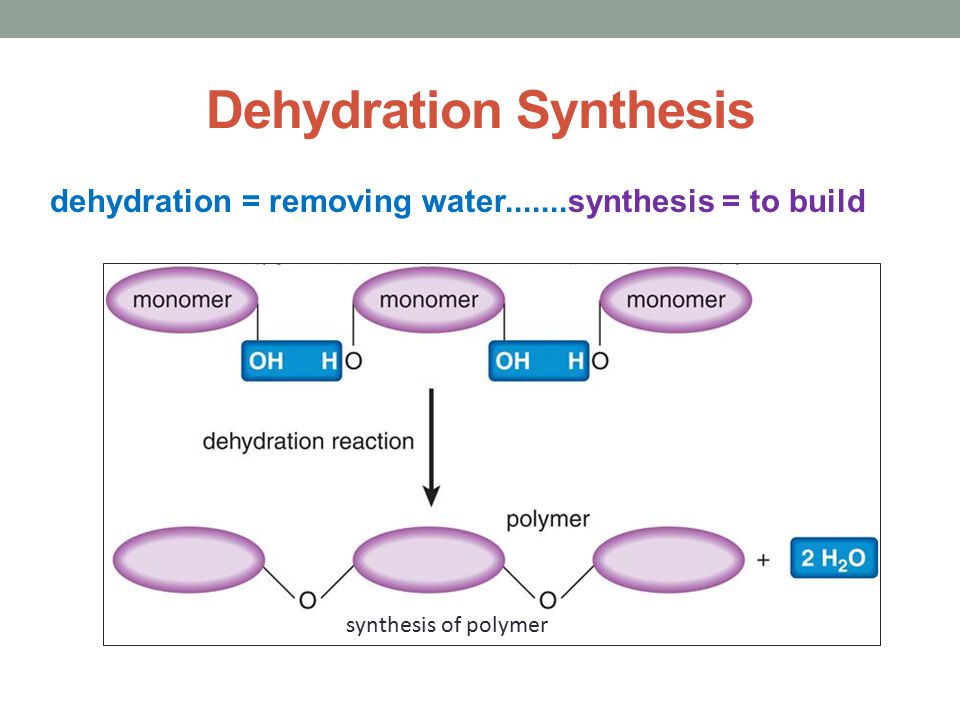
Which of the Following Chemical Equations Describes a Dehydration Reaction
Copper sulfate pentahydrate undergoes dehydration to produce anhydrous copper sulfate and five molecules of. The loss of water molecule can occur due to reaction between two functional groups like OH -NH 2 or COOH.
What Do The Following Equations Represent Ppt Download
Magnesium Oxygen Magnesium oxide.
. Monosaccharide monosaccharide disaccharide. Often such reactions require the presence of a dehydrating agent. A dehydration reaction or condensation reaction is the process in which.
Water molecules are produced as a polymer is formed from monomers Monomers are joined together in a reaction in which two molecules are covalently bonded to each other through the loss of a water molecule. The classic example of a dehydration reaction is the Fischer esterification which involves treating a carboxylic acid with an alcohol to give an ester. Carbonic acid H2CO3 is a weak acid.
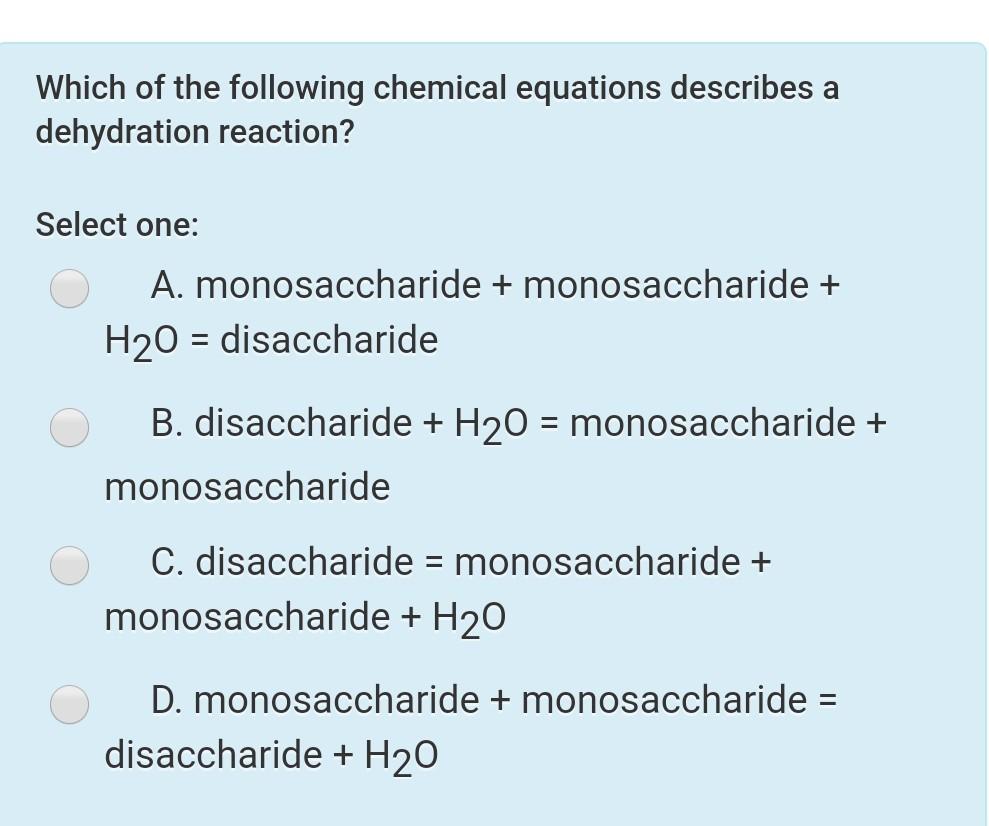
A disaccharide monosaccharide monosaccharide H2O B disaccharide H2O monosaccharide monosaccharide C monosaccharide monosaccharide disaccharide H2O D monosaccharide monosaccharide H2O disaccharide. Which of the following chemical equations describes a hydrolysis reaction. Dehydration Synthesis and Hydrolysis.
Which of the following chemical equations describes a hydrolysis reaction. Monosaccharide Monosaccharide Disaccharide H20 D. Which of the following chemical equations describes a dehydration reaction.
Disaccharide Monosaccharide Monosaccharide H2O B. Monosaccharide monosaccharide H20 disaccharide O B. AHydrolysis of simple sugars BHydrolysis of disaccharides CDehydration of polysaccharides DDehydration of simple sugars.
Dehydration reactions break down polymers and hydrolysis reactions create monomers. Dehydration reactions split water molecules and add hydroxyl groups to. Disaccharide monosaccharide monosaccharide H2O monosaccharide monosaccharide disaccharide H2O disaccharide H2O monosaccharide monosaccharide monosaccharide monosaccharide H2O disaccharide.
For example acetic acid CH 3 COOH forms acetic anhydride CH 3 CO 2 O and water by the dehydration reaction. A monosaccharide monosaccharide H2O disaccharide B monosaccharide monosaccharide disaccharide H2O C disaccharide monosaccharide monosaccharide H2O D disaccharide H2O monosaccharide monosaccharide. Reactions that produce acid anhydrides are dehydration reactions.
The four main categories of large biological molecules. Carbon dioxide CO2 is readily soluble in water according to the equation CO2 H2O----- H2CO3. Monosaccharide monosaccharide disaccharide H20.
Alkyl halides react with sodium dry ether to form higher alkanes. For example methyl bromide reacts with sodium in presence of dry ether to form ethane. If you were to describe a chemical reaction in a sentence it would be quite long.
Alkyl halides react with sodium in presence of dry ether to form higher alkanes. Methyl bromide 2 C H 3 B r 2 N a dry ether ethane C H 3 C H 3 2 N a B r b Finkelstein reaction. Dehydration synthesis involves the formation of new chemical bonds between two molecules which leads to the formation of new compounds.
Dehydration reactions assemble polymers and hydrolysis reactions break down polymers. The reaction between alkyl chlorides bromides and NaI dry. Dehydration reactions are also involved in the production of many polymers.
Which of the following chemical equations describes a dehydration reaction. 2 CH 3 COOH CH 3 CO 2 O H 2 O. Disaccharide monosaccharide monosaccharide H2O о O C.
Look at the word equation below. Disaccharide monosaccharide monosaccharide H20 D. Disaccharide H20 Monosaccharide Monosaccharide C.
Monosaccharide Monosaccharide H20 Disaccharide. Monosaccharide monosaccharide H20 disaccharide B. Which Of The Following Chemical Equations Describes A Dehydration Reaction.
If CO2 is bubbled into a beaker containing pure freshly distilled water which of the following graphs correctly describes the results. Which of the following best summarizes the relationship between dehydration reactions and hydrolysis. To express it in a shorter form we can write it as a word-equation.
A reaction occurs with the loss of water molecule at each step. Which of the following chemical equations describes a dehydration reaction. Heres the chemical equation that describes this reaction.
Sucrose water glucose fructoseWhich describes the chemical reaction taking place. Disaccharide H2O monosaccharide monosaccharide D. RCO 2 H ROH RCO 2 R H 2 O.
For example the reaction between magnesium and oxygen to give magnesium oxide can be represented as follows. Disaccharide H20 monosaccharide monosaccharide C. Reactants Product Substances.

Solved Which Of The Following Chemical Equations Describes A Chegg Com

Solved Which Of The Following Chemical Equations Describes A Chegg Com

Solved 1 2 3 4 5 6 7 8 9 10 Question Answer Choose The One Chegg Com
Comments
Post a Comment